Magnetecs CGCI Receives CE Marking for Sales in Europe
December 15, 2011
INGLEWOOD, Calif. – Magnetecs Corporation, a designer and manufacturer of robotic catheterization control systems for minimally invasive surgical procedures, today reported that the company’s Robotic Catheter Guidance Control and Imaging™ (CGCI) System has received CE Marking certification.
CE Marking will enable commercialization of CGCI in Europe and support certification in many other parts of the world. The scope of the indication for CGCI's CE Marking is "Design, manufacture and installation of CGCI System to navigate a magnetic device to designated target sites. Design and manufacture of Catheter Impedance Seeking Device (CISD) as an accessory to CGCI.”

“CE Marking is the key achievement of Magnetecs required to transition from the development phase of our organization to building a commercial enterprise,” said Josh Shachar, CEO of Magnetecs Corporation. “The scope of the indication we have received will enable widespread application of our technology for electrophysiology and interventional cardiology as well as additional fields of use.”
Below is the Magnetecs Regulatory Team, under the direction of David Johnson, Vice President and Director of Software Engineering, responsible for the CE Marking Certification.
MAGNETECS REGULATORY TEAM

STRONG CGCI SALES PIPELINE IN EUROPE
CE Marking will enable Magnetecs to generate revenue from sales in Europe in countries that include the U.K., Czech Republic, Sweden, Italy, Germany, Switzerland, and Russia.
Magnetecs is planning additional installations in North America in Montreal, Canada, Utah, and New York City in anticipation of the company’s FDA 510(k) submission.
ABOUT CGCI
CGCI is a unified system for robotic guidance, control and imaging of catheters and other advanced tools used in electrophysiological and other procedures. Previous magnetic guidance systems use large, independent magnets which emit a substantial and continuous magnetic field, have limited control capabilities, and require shielding. In contrast, Magnetecs’ CGCI system creates an electromagnetic field that is largely contained in the electromagnetic array and focused in an area no larger than the patient’s chest. CGCI only emits a magnetic field when in use and can dynamically adjust and manipulate this field to safely and reliably achieve unprecedented three-dimensional catheter-guidance precision and responsiveness.
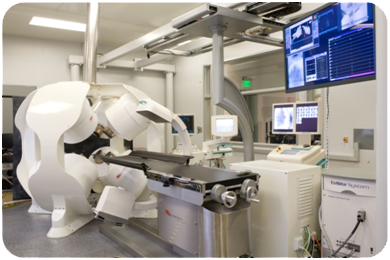 The Magnetecs’ Robotic CGCI
The Magnetecs’ Robotic CGCI
The CGCI system is integrated with sophisticated cardiac mapping and navigation technologies, including X-ray, intracardiac echocardiography (ICE), and other advanced electrophysiology lab technologies and equipment. Magnetecs is planning additional applications of the Company’s electromagnetic technology in the fields of interventional cardiology, gastroenterology, neurology, and gynecology.
ABOUT MAGNETECS
Magnetecs Corporation designs and manufactures a unique and highly efficient robotic catheterization control system for minimally invasive surgical procedures and the advanced specialized tools used in these procedures. The Company believes that its proprietary CGCI system will greatly improve the efficacy, safety and cost efficiency of many common minimally invasive surgeries. Magnetecs has established advanced electrophysiology surgical suites for CGCI development and testing at the Company’s headquarters facility in Inglewood, California.
For Further Information, Contact:
Daniel Saks, SVP, Corporate Affairs
(310) 670-7700 |