Magnetecs Recieves Grants Totaling $626,000 for Remote Navigation Systems
November 08, 2010
Inglewood, CA – Magnetecs Corporation, a designer and manufacturer of robotic systems for minimally invasive surgical procedures, today reported that the Company has received two grants to-date for a total of $626,000 for development of remote magnetic navigation systems.

The Company recently received a grant of $244,000 from The United States Internal Revenue Service Therapeutic Discovery Project Program, which was created by the Affordable Care Act. This grant is provided to US companies that have development projects showing significant potential to produce new cost-saving therapies, create U.S. jobs, and increase U.S. competitiveness.

Magnetecs has received a grant of up to $382,000 from the Israel-U.S. Binational Industrial Research and Development (BIRD) Foundation for a joint development project with Given Imaging Ltd, a world leading company from Israel in the field of gastroenterology, for a Magnetically Guided Capsule Endoscope.
“These grants represent confirmation of our vision to fulfill the promise of robotic surgery by increasing the efficacy, safety and efficiency of a wide range of minimally invasive procedures,” said Josh Shachar, CEO of Magnetecs Corporation. “As Magnetecs moves into the certification and pre-commercialization phase of our Robotic Catheter Guidance Control and Imaging system for electrophysiology, we are pleased to receive government and foundation support for this application and for our next application in gastroenterology."
CGCI HUMAN STUDIES ONGOING IN MADRID
Human studies using CGCI for patients with irregular heartbeat began on October 7, 2010 at Hospital General Universitario La Paz in Madrid, Spain. The studies are being conducted by Dr. Jose Merino, Director of the Arrhythmia & Electrophysiology Research Unit of the hospital. The Principal Investigator for the studies is Dr. Vivek Reddy, Professor of Medicine and Director, Cardiac Arrhythmia Service, Mount Sinai School of Medicine, in New York City. The studies will also be conducted by Dr. Eli Gang, Chief Medical Officer of Magnetecs, Clinical Professor of Medicine, Geffen School of Medicine at UCLA, who has also served as Director of the Clinical Electrophysiology Laboratory at Cedars-Sinai Medical Center in Los Angeles.
To-date 15 patients have participated in the first 20-patient study in which a highly detailed map of the heart is created using the CGCI system’s magnetically guided catheter. The primary outcome of the study measures intracardiac anatomic site target acquisition and repetition of acquisition. Successful outcomes have been achieved in all 15 patients thus far participating in the study. A description of the study can be found on the ClincalTrials.gov site.
The first mapping study is expected to be completed by the end of 2010 and will be followed by an additional mapping study of 20 patients. This second study is expected to be completed by the end of the first quarter of 2011. A subsequent study of 40 patients in which mapping and ablation procedures will be conducted using the CGCI system is expected to be completed in the second quarter of 2011.
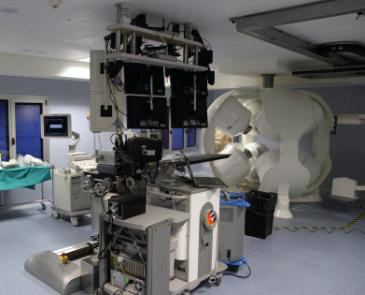
CGCI EP Suite at La Paz Hospital
CGCI SUBMISSION FOR CE MARK CERTIFICATION
CGCI uses eight electromagnets in a unique configuration to intelligently guide a magnetically-tipped catheter, enabling a physician to precisely and consistently control surgical tools in highly dynamic or previously inaccessible environments while enhancing both the physician’s dexterity and the patient’s safety. The first study in mapping of the heart is a diagnostic procedure that is performed for patients who have arrhythmia, or irregular heart beat. Magnetecs expects this study to lead to a CE Mark application for commercialization in Europe planned for the first half of 2011. Additional human studies for ablation are expected to lead to approval of the CGCI system for therapeutic procedures used to correct heart arrhythmia.
CGCI INSTALLATIONS PLANNED IN LONDON, NEW YORK CITY AND PRAGUE
Additional installations in Europe and the US will support the Company's program to receive FDA 510(k) certification. Magnetecs is currently in the initial stages of planning human studies in the US at Mount Sinai Medical Center in New York City under the direction of Dr. Reddy and Dr. Andre d'Avila, and in the UK at St. Mary’s Hospital in London under the direction of Dr. Nicholas Peters and Dr. Wyn Davies. CGCI installations and studies are also currently being planned at Na Homolce Hospital in Prague, Czech Republic.
ABOUT CGCI
CGCI provides a unified system for robotic guidance, control and imaging of electrophysiology and other procedures. The CGCI system is integrated with sophisticated cardiac mapping and navigation technologies, including intracardiac echocardiography (ICE), and other advanced electrophysiology lab technologies and equipment. Magnetecs is planning additional applications of the Company’s electromagnetic technology in the fields of interventional cardiology, gastroenterology, neurology, and gynecology.
ABOUT MAGNETECS
Magnetecs Corporation designs and manufactures a unique and highly efficient robotic catheterization control system for minimally invasive surgical procedures, and the advanced specialized tools used in these procedures. The Company believes that its proprietary Catheter Guidance Control and Imaging (CGCI) system will greatly improve the efficacy, safety and cost efficiency of many common minimally invasive surgeries. Magnetecs has established advanced electrophysiology surgical suites for CGCI development and testing at the Company’s headquarters facility in Inglewood, California.
For Further Information, Contact:
Daniel Saks, SVP, Corporate Affairs
(310) 670-7700 |