Magnetecs CGCI Robotic Ablation Procedure Presented At Boston AF Symposium
February 20, 2012
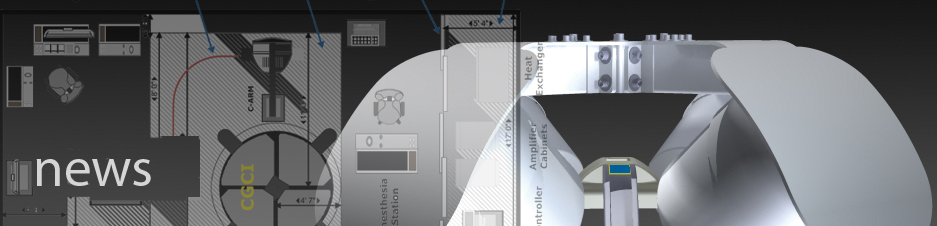
INGLEWOOD, Calif. – Magnetecs Corporation, a designer and Symposium on January 12, 2012. The case was performed by Dr. José Merino, Director of the Arrhythmia and Electrophysiology Robotics Research Unit at La Paz University Hospital in Madrid, Spain. The case can be viewed by clicking the video link below:“This case presents the first use of the CGCI system for ablation to correct arrhythmia in the left atrium and the first case presentation to a U.S. medical conference,” said Dr. Merino. Previously, a "live-case" study of the Magnetecs CGCI system was presented at the June 2011 EHRA Europace Congress in Madrid, Spain. This presentation consisted of a cardiac mapping procedure using the robotic CGCI system.CASE SUMMARYThe case presented at the Boston AF Symposium was a 67-year-old woman who has suffered from aortic valve regurgitation and recurrent atypical atrial flutter (AFL) since 2008. The patient’s condition was refractory to several antiarrhythmic drugs. The patient first underwent a conventional ablation procedure in 2010.(Above) Multiple views of a 'roof' lesion set. These overlapping RF lesions connect the left superior pulmonary vein to the right superior pulmonary vein. The atrial flutter was seen to terminate abruptly after completion of the line.In the Boston AF case presentation, the CGCI remote navigation system was used to map the left atrium (LA) and pulmonary veins and to create an accurate geometric representation of these structures. CGCI also enabled the creation of a detailed map of the activation pattern of the pathologic cardiac rhythm. The AFL terminated after creation of a line of conduction block between the two superior pulmonary veins, known as a “roof line”. Ablation was performed in the following manner: a “line” of overlapping ablation target sites was created by the physician just by clicking with a mouse on the surface of the 3D LA electroanatomical map. Thereafter, CGCI was commanded to reach each of these target sites in an “automated” mode, whereupon RF energy was delivered at each site. When the final ablation site was reached and energy delivered, the “roof line” was completed, and the rapid heart rhythm was seen to abruptly terminate.12-lead ECG of the patient's rhythm disturbance (left atrial flutter).Abrupt termination of atrial flutter upon completion of left atrial 'roof line' of RF lesions.CLINICAL TRIALS PERFORMED AND PLANNED USING CGCITo date, seven ablation procedures have been performed by Dr. Merino at La Paz University Hospital using CGCI. Of the seven ablation procedures, two were accessory pathway procedures, and five were performed on patients with atrial flutter. All seven procedures were performed with complete success. Dr. Merino and his team are preparing for a 40-patient ablation study testing the efficacy of CGCI for patients with atrial fibrillation. This study is currently planned to commence in April 2012.Close-up view of 'roof line' lesion set in the left atrium. A set of target lesions was delineated by the physician (light colored circles). The automated navigation feature then guided the catheter automatically to each site, whereupon RF energy was delivered (dark colored circles).In June 2011, Magnetecs announced the completion of a 40-patient clinical trial that consisted of mapping procedures at La Paz Hospital using CGCl. This trial validated the accuracy of mapping procedures using CGCI within the four chambers of the heart. In this study, in which the subsequent ablations were performed manually, each anatomic site was reached with manual magnetic mode using the CGCI joystick, then in automated mode. Excellent accuracy, in excess of 95%, was achieved in both modes, confirming the accuracy achieved in previous studies.The CGCI Robotic System with Control Room in BackgroundABOUT CGCICGCI is a unified system for robotic guidance, control and imaging of catheters and other advanced tools used in electrophysiological and other procedures. Previous magnetic guidance systems use large, independent magnets which emit a substantial and continuous magnetic field, have limited control capabilities, and require shielding. In contrast, Magnetecs’ CGCI system creates an electromagnetic field that is largely contained in the electromagnetic array and focused in an area no larger than the patient’s chest. CGCI only emits a magnetic field when in use and can dynamically adjust and manipulate this field to safely and reliably achieve unprecedented three-dimensional catheter-guidance precision and responsiveness.The CGCI system is integrated with sophisticated cardiac mapping and navigation technologies, including X-ray, intracardiac echocardiography (ICE), and other advanced electrophysiology lab technologies and equipment. Magnetecs is planning additional applications of the Company’s electromagnetic technology in the fields of interventional cardiology, gastroenterology, neurology, and gynecology.ABOUT MAGNETECSMagnetecs Corporation designs and manufactures a unique and highly efficient robotic catheterization control system for minimally invasive surgical procedures and the advanced specialized tools used in these procedures. The Company believes that its proprietary CGCI system will greatly improve the efficacy, safety and cost efficiency of many common minimally invasive surgeries. Magnetecs has established advanced electrophysiology surgical suites for CGCI development and testing at the Company’s headquarters facility in Inglewood, California.For Further Information, Contact: Daniel Saks, SVP, Corporate Affairs (310) 670-7700 |

|